News
Newswise —
Sudden Infant Death Syndrome (SIDS) is a rare and devastating condition that is a concern for all parents. SIDS claims the lives of about 3,500 babies each year in the United States, with the greatest risk in the first 12 months of life.
Recently, the American Academy of Pediatrics (AAP) issued a recommendation that infants sleep in their parents’ room, close to the parents’ bed — but on a separate surface designed for infants — for at least 6 months, and preferably up to 1 year of age. Such a sleeping arrangement decreases the risk of SIDS by as much as 50 percent, according to the AAP.
The AAP recommendations go on to recommend placing the crib/bassinet close to the parent’s bed so that the infant is within view and reach, which in turn will facilitate feeding, comforting, and monitoring the infant. Since these recommendations have come out, I have heard an uproar from fellow pediatricians’, parents and colleagues saying, “Really?” “What about the parent’s sleep and daytime performance?” “If these guidelines are followed isn’t the lack of sleep of the parents likely to cause more harm than good?”
What is interesting is that these are not new recommendations (this recommendation was also in the AAP’s 2011 guidelines); however, the wording is stronger and there is now an added timeline. So why the change?
One of the authors of the new guidelines has stated in an interview with the New York Times that the recommendations were updated “to be on the prudent side”. It is known that the first 6 months are a particularly critical time for infants because the rates of SIDS and other sleep-related deaths, particularly those occurring in bed–sharing situations, are highest in the first 6 months of life. In addition, while the rate of SIDS has plateaued over the last few years, the rates of other sleep-related infant deaths, such as accidental suffocation and strangulation in bed and ill-defined deaths have increased. Risk factors for these different categories of death are strikingly similar to those of SIDS. Therefore public health efforts are doubling down on environmental risks such as prone and side-sleep positioning, bed sharing, and soft bedding in hopes of decreasing these other types of sleep-related deaths.
Ultimately, this decision is a personal one, but understanding why this recommendation was made can help parents make an informed choice.
Following is a summary of the AAP’s recommendations for safe infant sleeping and to reduce the risk for SIDS. More complete information can be found here.• Place your baby to sleep on his back for every sleep.• Place your baby to sleep on a firm sleep surface.• Keep soft objects, loose bedding, or any objects that could increase the risk of entrapment, suffocation, or strangulation out of the crib.• Place your baby to sleep in the same room where you sleep but not the same bed.• Breastfeed as much and for as long as you can. This helps reduce the risk of SIDS.• Schedule and go to all well-child visits. Your baby will receive important immunizations.• Keep your baby away from smokers and places where people smoke. • Do not let your baby get too hot. • Offer a pacifier at nap time and bedtime. • Do not use home cardiorespiratory monitors to help reduce the risk of SIDS.• Do not use products that claim to reduce the risk of SIDS.
Learn more about Valley’s Pediatric Sleep Disorders and Apnea Center.
Newswise —
Virginia Tech scientists have developed a new cancer drug that uses gold nanoparticles created by the biotech firm CytImmune Sciences to deliver paclitaxel — a commonly used chemotherapy drug directly to a tumor.
Because of the direct targeting, the new effort not only increases the effectiveness of paclitaxel, it also dramatically reduces devastating side effects such as hair loss, nausea, and nerve pain.
CytImmune earlier this year asked David Kingston, a University Distinguished Professor of Chemistry with the Virginia Tech College of Science, to create a paclitaxel derivative that binds to gold-based nanoparticles while in the blood stream, only releasing the drug once it’s inside a cancerous tumor. Paclitaxel chemotherapy is widely used to treat breast, ovarian, lung, and colon cancer.
“Paclitaxel side effects occur because the drug is given intravenously and thus is distributed throughout the body, and not just to the tumor,” said Kingston, who joined the Virginia Tech Department of Chemistry in 1971. “In addition, the solvent used to allow infusion has its own toxicity. Paclitaxel could be a much more effective drug if it could be targeted directly to the tumor. This would allow each dose to be given without causing significant side effects, and would thus increase the potential for cures.”
In other words, for now, delivery of a paclitaxel equals a shotgun with pellets. The blast of killing a tumor results in great collateral damage. Kingston and his team say their delivery method is like a finely tuned rifle, using CytImmune’s gold-based nanoparticles as the delivery bullet.
The gold nanoparticles are decorated with both paclitaxel and tumor necrosis factor – a cell-signaling protein commonly called TNF. Gold nanoparticles are known to cling around cancerous tumors. TNF thus binds to the tumor blood vessel cells, ultimately killing them and reducing the high pressure inside the tumor, which prevents paclitaxel from reaching the cancer cells to kill them.
Now, the slowly released paclitaxel that is bound to the gold nanoparticles can reach its targeted cancer cells to kill them.
In early lab tests in treating mouse melanoma, a 2.5 milligram dose of paclitaxel delivered on Kingston’s gold nanoparticles vehicle was essentially as effective as a dose of 40 milligrams of paclitaxel by itself.The delivery method is expected to soon move toward clinical trial, said Kingston.
Findings by Kingston and his team – including Jielu Zhao, a 2016 doctoral graduate in chemistry, now a chemist at Proctor and Gamble, and Shugeng Cao, a former post-doctorate researcher also in chemistry, now an associate professor at the University of Hawaii at Hilo — were recently published in the scientific journal Bioconjugate Chemistry.
Zhao and Cao carried out the actual synthesis of the paclitaxel derivatives with the designed linkers to allow them to bond to the gold nanoparticles, with Kingston supervising.
“This approach has the potential to be a game-changer in nanoparticle-based drug delivery systems,” said Kingston, “since it combines the power of drug targeting by tumor necrosis factor, with the advantages of nanoparticle delivery, including the low toxicity of nanoparticle drugs to normal, healthy tissue.”
“By combining the tumor blood vessel destroying activity of TNF with the cancer killing effect of paclitaxel onto CytImmune’s tumor-targeted, ‘stealth’ gold nanoparticles, Dr. Kingston’s team and CytImmune’s team may have potentially created a new cancer drug that is far more effective and less toxic to the human body,” said Lawrence Tamarkin, chief executive officer at CytImmune.
Work on the new drug was split between Virginia Tech’s main Blacksburg campus and CytImmune’s Rockville, Maryland, headquarters. Kingston has teamed with CytImmune in the past on tumor-targeting nanomedicine.
Virginia Tech previously has used gold nanoparticles in unrelated anti-cancer research, including the Virginia-Maryland College of Veterinary Medicine which in experiments used gold nanoparticles to collect around tumors found inside a dog, and then utilized a non-ablative laser to target the gold nanoparticles, and thus the tumors. In essence, the veterinary approach killed cancer cells by heating them, versus Kingston’s approach directly targeting paclitaxel to tumors via the gold nanoparticles.
Newswise —
An experimental drug that targets abnormally high levels of a protein linked to cancer growth appears to significantly reduce the proliferation of prostate cancer cells in laboratory cell cultures and animals, while also making these cells considerably more vulnerable to radiation, according to results of a study led by Johns Hopkins scientists.
The findings, published Sept. 12 in Cancer Research, could advance the search for novel combination treatments that make more effective and safer use of radiation against prostate cancer, the most common nonskin cancer in men and the second leading cause of cancer-related deaths in men in the United States.
Of the nearly 200,000 men diagnosed with prostate cancer each year in the United States, radiation is a first-line therapy considered for all but the most advanced disease. However, some of these cancers become resistant to the effects of radiation over time, according to Venu Raman, Ph.D., an associate professor of radiology and radiological science and of oncology at the Johns Hopkins University School of Medicine and member of the Johns Hopkins Kimmel Cancer Center.
In a search for ways of extending the value of radiation and limiting the collateral damage to healthy tissue that necessarily high doses of radiation may inflict, Raman worked with Phuoc Tran, M.D., Ph.D., an associate professor of radiation oncology and molecular radiation sciences, oncology, and urology, and also a member of the Kimmel Cancer Center.
They and colleagues from Johns Hopkins and University Medical Centre Utrecht had earlier discovered that a protein called DDX3 appears to be "dysregulated" in many cancers, including breast, lung, colorectal, sarcoma and prostate. The researchers found that the more aggressive the cancer, the higher the expression of this protein, which helps maintain cellular stability.
The researchers then developed a molecule referred to as RK-33 that was designed to disrupt DDX3's function by locking on to a portion of the protein. They showed in previous studies with cell cultures that when adding RK-33 to malignant lung and other cancerous cells that highly express DDX3, proliferation slowed or halted, and the cells' ability to form colonies was impaired. Additionally, RK-33 appeared to be a radiosensitizer, making the destructive effects of radiation more pronounced.
In the new study, the researchers began by examining prostate cancer tissue samples from University Medical Centre Utrecht. Of the 23 samples with a Gleason score greater than seven, eight had high DDX3 expression.As with results of their earlier studies, the investigators found that the higher the expression of this protein, the more aggressive the cancer, which is determined by how the cells invade other tissue types and their ability to form tumors in laboratory models of cancers. When the researchers used gene engineering techniques to knock out DDX3 expression in laboratory-grown cell cultures that highly expressed this protein, cell proliferation was half that of cell cultures with high DDX3 expression.
Incubating cultured cells with RK-33 had a similar effect, knocking down DDX3 expression in cells that highly express this protein and hampering their ability to multiply. When researchers combined the drug with radiation, the effects were synergistic, they report, killing from two to four times more cells than radiation alone.Next, the researchers tested the effects of RK-33 and radiation in mice that had been injected with human prostate cancer cells that highly express DDX3. The animals formed tumors within a few weeks. Together, Raman says, this dual-mode treatment produced cell-killing results that paralleled their experiments in cell cultures.
Raman adds that the experimental drug appeared to have no toxicity in the mice, suggesting that it could be a promising drug to test in humans. Compounds based on RK-33, he says, might have value in treating a broad array of cancers that highly express DDX33 or as a supplement to radiation, making conventional doses more effective or improving the killing ability of lower doses."A lot of work still needs to be done to develop this into a chemotherapy drug," Raman cautions. "But based on our findings, we think it could fill an unmet need in making the most common treatment for prostate cancer more effective."
Other Johns Hopkins researchers who participated in this study include Min Xie, Farhad Vesuna, Saritha Tantravedi, Guus M. Bol, Marise R. Heerma van Voss, Katriana Nugent, Reem Malek and Kathleen Gabrielson.
SEE ORIGINAL STUDY
Loyola University Medical Center
Erika Fleck is seizure free following epilepsy surgery.
Newswise — MAYWOOD, IL –
By the time epilepsy patient Erika Fleck came to Loyola Medicine for a second opinion, she was having three or four seizures a week and hadn’t been able to drive her two young children for five years.
“It was no way to live,” she said.
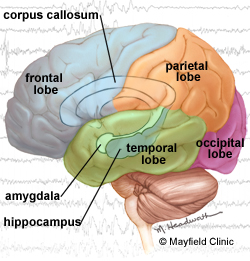
Loyola epileptologist Jorge Asconapé, MD, recommended surgery to remove scar tissue in her brain that was triggering the seizures. Neurosurgeon Douglas Anderson, MD, performed the surgery, called an amygdalohippocampectomy. Ms. Fleck hasn’t had a single seizure in the more than three years since her surgery.
“I’ve got my life back,” she said. “I left my seizures at Loyola.”
Surgery can be an option for a minority of patients who do not respond to medications or other treatments and have epileptic scar tissue that can be removed safely. In 60 to 70 percent of surgery patients, seizures are completely eliminated, and the success rate likely will improve as imaging and surgical techniques improve, Dr. Anderson said.
Traditionally, patients would have to try several medications with poor results for years or decades before being considered for surgery, according to the Epilepsy Foundation. “More recently, surgery is being considered sooner,” the foundation said. “Studies have shown that the earlier surgery is performed, the better the outcome.” (Ms. Fleck is a service coordinator for the Epilepsy Foundation North/Central Illinois Iowa and Nebraska.)
Dr. Asconapé said Ms. Fleck was a perfect candidate for surgery because the scar tissue causing her seizures was located in an area of the brain that could be removed without damaging critical structures.
Ms. Fleck experienced complex partial seizures, characterized by a deep stare, unresponsiveness and loss of control for a minute or two. An MRI found the cause: A small area of scar tissue in a structure of the brain called the hippocampus. The subtle lesion had been overlooked at another center.
Epilepsy surgery takes about three hours, and patients typically are in the hospital for two or three days. Like all surgery, epilepsy surgery entails risks, including infection, hemorrhage, injury to other parts of the brain and slight personality changes. But such complications are rare, and they pose less risk to patients than the risk of being injured during seizures, Dr. Asconapé said.
Loyola has been designated a Level Four Epilepsy Center by the National Association of Epilepsy Centers. Level Four is the highest level of specialized epilepsy care available. Level Four centers have the professional expertise and facilities to provide the highest level of medical and surgical evaluation and treatment for patients with complex epilepsy.
Loyola's comprehensive, multidisciplinary Epilepsy Center offers a comprehensive multidisciplinary approach to epilepsy and seizure disorders for adults and children as young as two years old. Pediatric and adult epileptologist consultation and state-of-the-art neuroimaging and electrodiagnostic technology are used to identify and assess complex seizure disorders by short- and long-term monitoring.
The Epilepsy Foundation provides individual and family assistance, advocacy and epilepsy education. For more information, call (815) 964-2689.
Newswise — ANN ARBOR, Mich. —
To get the best results for patients, it may pay for their doctors to heed the words of legendary University of Michigan football coach Bo Schembechler: the team, the team, the team.
A new study finds that heart surgery patients’ chances of survival depends in part on the overall level of teamwork among all the physicians who cared for them across their surgery preparation, operation, hospitalization and recuperation.
The finding was made by a team of researchers at the U-M Medical School, just a few miles away from the stadium where Schembechler once coached.
Using data from 251,000 older Americans who had heart bypass surgery, they mapped the interactions among the 466,000 doctors who cared for them, and show the importance of tight “social networks” of physicians to patient outcomes.
They’ve published their results in the new issue of Circulation: Cardiovascular Quality and Outcomes, and hope to test their model in other conditions and by considering other types of providers beyond physicians. They’re already studying the effect further, using anthropology research techniques in the U-M Comprehensive Cancer Center.
“Surgical care is complex, involving multiple providers dispersed across locations over time,” says John Hollingsworth, M.D., M.S., lead author of the new paper and a urologist at U-M. “Our findings show that physician teamwork influences patient outcomes, even more than some measures of comorbid illness.”
The more often their physicians worked with one another in the past on the care of other patients, the lower their current patients’ chances were of ending up in the emergency department or hospital, and their chances of dying, within the first two months after their operation.
Measuring teamwork
The researchers created their model of physician teamwork based on Medicare data that showed which physicians from nearly all disciplines had treated the same patients 30 days before and 60 days after their hospitalization for surgery. They factored in measures of the social and healthcare environment in the communities where the bypass surgery hospitals were located, and data on each patient’s 60-day outcomes.
The results were striking. A 25 percent increase in the measure of teamwork was associated with 17 fewer readmissions for every 1,000 patients treated.
That’s only slightly less impact than a similar-size increase in a measure of education in that same regional community; higher levels of education are already known to be associated with better surgical results. A 25 percent increase in a measure of how complex and sick patients are – called a Charlson score -- only accounted for one extra readmission per 1,000 patients.
Wide variation
Hollingsworth and his colleagues, including senior author and U-M cardiologist Brahmajee Nallamothu, M.D., MPH, note that regions varied widely in their teamwork ‘score’. To illustrate, their paper shows a map of ties among physicians who treated patients who had their operations in 2011 in two Texas hospitals 90 miles apart -- one in Waco and the other Fort Worth.
The measure of teamwork among all the physicians who treated these patients across the eight-month span surrounding their operation was nearly five times higher among Waco physicians than among Fort Worth physicians. The Fort Worth doctors rarely shared more than one patient in common, while a core set of Waco physicians shared many patients in common, with other physicians interacting less often.
The teamwork effect persisted no matter how large or small the number of bypass operations performed at a given hospital, or the type of hospital. Large academic medical centers tended to have lower teamwork scores because they receive far more patients who are referred to them from physicians far outside their local area who they don’t work with often. This may help explain why those hospitals don’t always score high on Medicare’s measures of patient outcomes.
“A lot of the focus in improving care is focused on the acute hospitalization for an episode of care. We believe that this focus is too myopic because it ignores the care delivered prior to the hospital stay and after discharge,” says Hollingsworth. “Efforts to improve teamwork, and outcomes, need to consider the entire care continuum.”
Hollingsworth and Nallamothu are both members of the U-M Institute for Healthcare Policy and Innovation. They worked with U-M cardiac surgeon Frank Pagani, M.D., Ph.D., and U-M sociologist Jason Owen-Smith, Ph.D., as well as Russell Funk, Ph.D., who earned his economic sociology degree at U-M and now is at the University of Minnesota. Their work was funded by the Agency for Healthcare Research and Quality (HS020927).
The researchers are now hoping to look at other data from Medicare and beyond to see if the teamwork effect has a similar impact on outcomes in other types of care, and in care covered by other forms of insurance. Because only physician names are captured in the bills sent to Medicare, they will need to look for other ways of assessing the impact of teamwork among nurses, nurse practitioners, physician assistants, physicians still in their residency training and front-end staff.
New funding from the National Science Foundation is fueling their studies of how networks evolve on the ground level in medicine, by observing the interactions among physicians who share patients. By understanding what factors influence physicians’ ties, and the flow of patients among them, says Hollingsworth, they hope to point out opportunities to strengthen teamwork and improve patient outcomes. The effort has started in the U-M prostate cancer clinic, and the team is looking for other clinics to join in.
Bo Schembechler may have died 10 years ago this month, but his mantra “the team, the team, the team” may be one that makes as much difference in medicine as it does in football.
Reference: Circulation: Cardiovascular Quality and Outcomes, DOI: 10.1161/CIRCOUTCOMES.116.002714 # # #
Newswise —
Some 10 million points of genetic variation are scattered across a molecule of DNA, and those variations make us who we are as individuals. But in some cases, those variants contribute to diseases, and it’s a major challenge for scientists to distinguish between harmless variants and those that are potentially hazardous to our health.
Now, researchers at Washington University School of Medicine in St. Louis have developed a new technique to cheaply and rapidly create myriad sets of DNA fragments that detail all possible genetic variants in a particular stretch of DNA. By studying such DNA fragments, scientists can more easily distinguish between genetic variants linked to disease and those that are innocuous.
The findings, published Oct. 3 in Nature Methods, allow researchers to create sets of DNA variants in a single day for a few hundred dollars. Current methods take up to a week and cost tens of thousands of dollars.
“As a pediatric neurologist who does a lot of genetic studies of kids with developmental disabilities, I frequently will scan a patient’s whole genome for genetic variants,” said Christina Gurnett, MD, PhD, the study’s senior author and an associate professor of neurology and of pediatrics. “Sometimes I’ll find a known variant that causes a particular disease, but more often than not I find genetic variants that no one’s ever seen before, and those results are very hard to interpret.”
In the past, scientists tested the effect of genetic variants one by one, a laborious process. At a single point in the DNA sequence, they replaced the correct DNA letter – an A, T, C or G – with one of the other three options. Then, they translated that DNA sequence into a protein and evaluated whether the mutated protein behaved differently than the original one.
More recently, researchers have begun creating sets of hundreds of variants in which each letter in a particular DNA sequence is changed, and then testing the set all at once. Such studies have been limited, however, by the high cost of creating those sets.
Postdoctoral researcher Gabriel Haller, PhD, who was working in Gurnett’s lab, realized that he could harness common laboratory techniques and tools to create sets of DNA variants without the expensive equipment and reagents that drove up the price.
Haller copied a DNA sequence using the four standard DNA letters and a nonstandard letter known as inosine. Each copy of the sequence was identical except for one inosine, which was located at a random spot and served as a placeholder. Then, he replaced the inosine with one of the standard DNA letters, creating a single mutation in each copy of the sequence.
Gurnett and colleagues are applying this technique to genes associated with aortic aneurysms, a weakening and ballooning of the aortic wall that can be fatal. Over the long term, Gurnett envisions the creation of a catalog listing the effects of every possible variant. The speed and cheapness of the new technique make such a catalog possible.
“Then, when clinicians find a variant that’s never been seen before in one of these genes associated with aortic aneurysm, they can go through this catalog and say, ‘Yes, this mutation does have a negative effect on that protein, so it’s likely harmful’,” Gurnett said. “It would help them decide what to tell the patient. This would be one piece of the big interpretation puzzle for genetic mutations.”
Newswise — ANN ARBOR, Mich. — For most invading bacteria, the bladder is not a friendly place.
But for those that have figured out how to scavenge iron from their hosts, it’s a fine place to grow and reproduce. And for millions of women a year, that means painful, burning, potentially dangerous urinary tract infections.
Now, in an ironic twist, scientists have turned that iron-scavenging power against the most common UTI-causing bacteria.
For the first time, they’ve prevented UTIs in mice by vaccinating them with the same molecules that the bacteria usually use to grab iron and fuel their growth. The results are published in the Proceedings of the National Academy of Sciences by a team from the University of Michigan Medical School.
They caution that a human UTI vaccine based on the approach is still years away. But the success of vaccination with the small iron-grabbing molecules, called siderophores, paves the way for further research.
The same team previously reported success in preventing UTIs using a vaccine made of proteins from the bacteria, called uropathogenic Escherichia coli or UPEC. Neither the protein approach nor the siderophore approach provided complete protection, but the two approaches together might.
UPEC causes more than three-quarters of UTIs in otherwise healthy women, and the bacteria produce “stealth” siderophores that evade the host immune system and are unique to them. That makes these molecules good candidates for a vaccine without unintended consequences.
In fact, in the same issue of PNAS, a team from the Massachusetts Institute of Technology and University of California, Irvine report success in preventing gut infections by vaccinating mice against Salmonella using siderophores made by those bacteria.
A pressing problem
With E. coli gaining resistance to the antibiotics that many women take to treat UTIs, or prevent recurring ones, the search for a vaccine takes on new urgency, says Harry Mobley, Ph.D., senior author of the new paper and chair of the Department of Microbiology and Immunology at U-M.
Recent findings that cranberry juice does not prevent UTIs adds to that urgency.
UTI care in the United States costs more than $3.5 billion a year, and affect half of all women sometime in their lives. Half of those who get one will get another in the next year, and about 4 million women suffer continuous UTIs. Women suffering recurrent UTIs are the most likely candidates for a vaccine, says first author and research fellow Laura Mike, Ph.D.
Mike, Mobley and their colleagues assessed the effect of siderophore vaccination in several ways. They started by applying two different UPEC stealth siderophores inside the noses of mice, several times over the course of two weeks.
A week later, they applied UPEC bacteria directly into the mouse bladders, waited two days, and then looked at their urine, bladder and kidneys, which get involved when a UTI rages out of control.
When each of the two siderophores was administered individually, modest protection against UTI resulted. But treating the mice with both siderophores and the carrier protein at the same time produced a much greater effect.
“We saw efficacy more in the kidney than in the bladder, suggesting that this approach may be most useful in preventing advanced UTIs,” says Mike. “Our next challenge is creating a combination vaccine that also employs proteins that UPEC bacteria use to bind their iron-laden siderophores, and test other adjuvants to increase the response.”
The team is planning to expand their study to see if they can protect against the bloodstream infection, or bacteremia, that can cause some UTI sufferers to develop sepsis and die.
Mobley notes that the researchers did not find direct evidence of antibodies made by the mice responding to the siderophore vaccine. But the findings, including detailed examination of the bladder and kidney tissue, are consistent with an adaptive immune response stemming from the vaccination, he says.
Other teams have tried to use siderophores against UTIs, but did not go as far as the U-M team. Using a small molecule such as a siderophore against bacterial infections is itself a novel approach, Mobley notes.
And, says Mike, since other dangerous bacteria make their own unique siderophores, the approach could be attempted beyond UPEC and Salmonella. Recently, U-M researchers showed the role of siderophores in the ability of a “superbug” bacteria called Klebsiella pneumonia to cause pneumonia and much worse.
“Using proteins that are found on the surface of bacterial cells as the basis for vaccination may lack efficacy because of the variability of the exact protein structure,” says Mobley. “But siderophores are the same across most gram negative enteric bacteria that cause some of the worst infections. This is a step along the journey, but it’s an encouraging one.”
In addition to Mobley and Mike, the study’s authors are Sara N. Smith, Christopher A. Sumner and Kathryn A. Eaton, Ph.D., DVM. The researchers used the U-M Medical School’s Proteomics and Peptide Synthesis Core, and the resources of the Host Microbiome Initiative launched by the school several years ago to support work on microbes that reside in and invade the human body.
The research was funded by the National Institutes of Health (AI116791, DK097362, AI007528) and by Mike’s support from a Research Scholars Fellowship from the American Urological Association administered by the Urology Care Foundation. Mobley is the Frederick G. Novy Distinguished University Professor of Microbiology and Immunology.Reference: PNAS Early Edition, DOI:10.1073/pnas.1606324113 http://www.pnas.org/cgi/doi/10.1073/pnas.1606324113# # #
SEE ORIGINAL STUDY
Newswise — DURHAM, N.C. --
Scientists at Duke Health and Zhejiang Chinese Medical University have developed a strategy to stop the uncontrollable itch caused by urushiol, the oily sap common to poison ivy, poison sumac, poison oak and even mango trees.
The team found that by blocking an immune system protein in the skin with an antibody, they could halt the processes that tell the brain the skin is itchy. The research was done in mice and is described in the Nov. 7 Proceedings of the National Academy of Sciences. They hope their model could lead to potential treatments for people who are allergic to poison ivy -- an estimated 80 percent of the population.
For most people, contact with poisonous plants is painful but not life-threatening. Still, there are significant health care costs associated with more than 10 million people in the U.S. affected each year, said senior author Sven-Eric Jordt, Ph.D., associate professor of anesthesiology at Duke.
“Poison ivy rash is the most common allergic reaction in the U.S., and studies have shown that higher levels of carbon dioxide in the atmosphere are creating a proliferation of poison ivy throughout the U.S. -- even in places where it wasn’t growing before,” Jordt said. “When you consider doctor visits, the costs of the drugs that are prescribed and the lost time at work or at school, the societal costs are quite large.”
Some symptoms of the fiery, blistering rash can be alleviated with antihistamines and steroids. But in recent years, scientists have determined that the most severe itching doesn’t go away with antihistamines, because it arises from a different source, Jordt said.
Jordt and collaborators determined the itch is triggered by interleukin 33 (IL-33), a protein in the skin involved in immune response.
All people have IL-33 in their skin, but the protein is elevated in people who haveeczema and psoriasis, Jordt said. The protein is known for inducing inflammation, but these new experiments show the protein also acts directly on the nerve fibers in the skin, exciting them and telling the brain that the skin is severely itchy.
The researchers used an antibody to block IL-33 and found that it not only reduced inflammation, but also cut down scratching in mice with poison ivy rashes. An antibody that counteracts human IL-33 is currently being evaluated in humans through a Phase 1 clinical trial to determine its safety and potential side effects.
In an additional approach tested in the mouse experiments, the researchers also found they could also alleviate itch by blocking a receptor for IL-33, called ST2.
“There could be translational significance here,” Jordt said. “So our next step will be to look at human skin to see if we see the same activity and the same pathways. We will also look at anti-inflammatory drugs that are already approved to see if they have the potential to alleviate itch.”
In addition to Jordt, study authors include Boyi Liu; Yan Tai; Satyanarayana Achanta; Melanie M. Kaelberer; Ana I. Caceres; Xiaomei Shao; and Jianqiao Fang.
The research was supported by the Duke Anesthesiology DREAM Innovation Grant (2015-DIG LIU), Zhejiang Chinese Medical University Start-Up Funding (722223A08301/ 001/004), the National Natural Science Foundation of China (81603676) and three National Institutes of Health -- the National Center for Advancing Translational Sciences (UL1 TR001117), the National Institute of Environmental Health Sciences (R01 ES015056, U01 ES015674) and the National Institute of Arthritis and Musculoskeletal and Skin Disease (R21 AR070554). The authors declare no conflicts of interest.###
SEE ORIGINAL STUDY
Newswise — Denver —
A new cell phone app specializing in sports injury detection captured 99 percent more physical and mental health symptoms for college athletes than traditional sports medicine surveillance, according to new research released today at the American Public Health Association’s 2016 Annual Meeting and Expo in Denver.
Researchers sampled more than 100 college football and cross-country athletes at three NCAA Division I universities during 2015 with a smartphone-based application used to collect health data in athletes’ natural environments. They found over 99 percent of the health symptoms obtained would not have been captured through traditional injury surveillance that relies on electronic medical records or clinician reports.
“These initial results are striking and provide important insight as to how we may be able to better interface athletes with the sports medicine team in the college setting,” said lead researcher Christine Baugh, MPH, Harvard University. “The ultimate goals would be to improve the health care received by college athletes and also to make injury and symptom surveillance more robust in these populations.”
According to Baugh, the data gathered during the study will be used to evaluate whether stress, sleep and head impacts sustained through sport influence athletes’ symptom patterns.
“We hope to use this information to provide a more holistic and accurate picture of how sports participation affects collegiate athlete health and wellbeing,” Baugh said.
APHA 2016 is themed “Creating the Healthiest Nation: Ensuring the Right to Health” and will focus on moving toward health equity, which means we must value all people equally, promote prevention and zero in on the social determinants of health.
3039.0: Smartphone-based Ecological Momentary Assessment: Pilot Test of Use in Evaluating Collegiate Athlete HealthDate: Monday, Oct. 31, 2016Researchers: Christine Baugh, MPH, Harvard University; Emily Kroshus, ScD, MPH, University of Washington; William Meehan, MD, Boston Children's HospitalInformation for media: The APHA Press Office will be located in room 604 of the Colorado Convention Center.
# # #
Newswise —
By the time unambiguous signs of memory loss and cognitive decline appear in people with Alzheimer’s disease, their brains already are significantly damaged, dotted with clumps of a destructive protein known as amyloid beta. For years, scientists have sought methods and clues to help identify brain changes associated with Alzheimer’s earlier in the disease process, so they can try to stop or even reverse the changes before they severely affect people’s lives.
Now, researchers at Washington University School of Medicine in St. Louis have developed a chemical compound, named Fluselenamyl, that detects amyloid clumps better than current FDA-approved compounds. If a radioactive atom is incorporated into the compound, its location in a living brain can be monitored using positron emission tomography (PET) scans.
The compound, described in a paper published Nov. 2 in Scientific Reports, one of the Nature journals, potentially could be used in brain scans to identify the signs of early-stage Alzheimer’s disease or to monitor response to treatment.
“Fluselenamyl is both more sensitive and likely more specific than current agents,” said Vijay Sharma, PhD, a professor of radiology, of neurology and of biomedical engineering, and the study’s senior author. “Using this compound, I think we can reduce false negatives, potentially do a better job of identifying people in the earliest stages of Alzheimer’s disease and assess the effects of treatments.”
Amyloid plaques are one of the most telltale findings in the brains of people with Alzheimer’s disease. The neurons near such plaques are often dead or damaged, and this loss of brain cells is thought to account for difficulty with thinking, memory loss and confusion experienced by Alzheimer’s patients.
Amyloid plaques can be either diffuse or compact. The compact kind has long been associated with the disease, but conventional wisdom has held that diffuse plaques are benign, since they can be found in the brains of elderly people without any symptoms of Alzheimer’s disease, as well as the brains of those with Alzheimer’s. Sharma believes that diffuse plaques may mark the earliest stages of the disease.
“It is a relatively underexplored area in the development of Alzheimer’s pathology,” Sharma said. “Since current approved agents don’t detect diffuse plaques, there is no reliable noninvasive imaging tool to investigate this aspect in animal models or in patients. Our compound could be used to study the role of diffuse plaques.”
Using human amyloid beta proteins, Sharma and colleagues showed that Fluselenamyl bound to such proteins two to 10 times better than each of the three FDA-approved imaging agents for detecting amyloid beta. In other words, Fluselenamyl detected much smaller clumps of the protein, indicating that it may be able to detect the brain changes associated with Alzheimer’s disease earlier.
To determine whether Fluselenamyl can detect plaques in the brain, the researchers used the compound to stain brain slices from people who had died of Alzheimer’s disease and, as controls, people of similar ages who had died of other causes. The brain slices from the Alzheimer’s patients, but not the controls, were identified as containing plaques.
When a radioactive atom was incorporated into the compound, the researchers found very little interaction between Fluselenamyl and the healthy white matter in the human brain slices.
“A huge obstacle with existing state-of-the-art PET agents approved for plaque detection is that they tend to bind indiscriminately to the brain’s white matter, which creates false positives on the scans,” Sharma said. Nonspecific binding to other parts of the brain creates “noise,” which makes it difficult to distinguish samples with plaques from those without.
A similar experiment comparing mice genetically predisposed to develop amyloid plaques with normal control mice showed the same pattern of high sensitivity for amyloid beta and low binding to healthy white matter.
Furthermore, Sharma and colleagues showed that when Fluselenamyl with the radioactive atom is injected intravenously into mice, the compound can cross the blood-brain barrier, bind to any plaques in their brains and be detected by PET scan. In mice without plaques, the compound is quickly flushed from the brain and then excreted from the body.
The next step is to move to testing in patients. Sharma already has submitted an application to the National Institutes of Health (NIH) for a phase 0 trial, to establish whether Fluselenamyl is safe for use in humans and behaves in the human body the same way it behaves in mice. Phase 0 trials involve a low dose given to a small number of people to learn how a molecule is processed in the body and how it affects the body.
“Ideally, we’d like to look at patients with very mild symptoms who are negative for Alzheimer’s by PET scan to see if we can identify them using Fluselenamyl,” Sharma said. “One day, we may be able to use Fluselenamyl as part of a screening test to identify segments of the population that are going to be at risk for development of Alzheimer’s disease. That’s the long-term goal.”