News
Newswise — GAINESVILLE, Fla. ---
Most people learn how to cook and safely handle food from their parents. Then they pass along their food knowledge and behaviors – right or wrong – from generation to generation. This cycle may prevent young people from learning all they can about food safety, a new University of Florida Institute of Food and Agricultural Sciences study shows.
But the UF/IFAS researcher leading the study says the findings present teachable moments. Joy Rumble and her research colleagues suggest more interactive and online instruction in food safety procedures, supplemented by social media outreach.
The real issue, as Rumble found in her newly published study, is that few Floridians bother to find out the safest ways to prevent food-borne illnesses.
And it’s not that they don’t care, said Rumble, an assistant professor in agricultural education and communication. “They’ve just never had a reason to care. They don’t know they are doing something wrong, or they’ve never knowingly gotten sick from something they made.”
In the study, Rumble led a team of UF/IFAS researchers that conducted an internet survey of 511 Floridians. They wanted to know if there’s a correlation between food safety behaviors, generations of Floridians and where Floridians learn about food safety behaviors.
They divided the respondents into age groups: millennials or younger (ages 20 to 39); those in Generation X (ages 40 to 51), young baby boomers (ages 52 to 61), older baby boomers (ages 62 to 70) and the silent generation (ages 71 and older).
One area researchers asked about was whether respondents disinfect counters before they get food ready to eat or cook. The study found that more than 70 percent of the millennials through old baby boomers do this, while 55 percent of the silent generation do this.
On the other hand, 79 percent of the silent generation properly defrosted frozen food in the refrigerator or microwave, while 40 percent of millennials reported doing this.
What millennials don’t know about proper food preparation stems partly from the convenience-driven society in which they’ve grown up. That includes ready-to-eat meals or meals cooked outside the home. Another factor for millennials and other age groups: home-economics classes.
Home economics was a fixture in secondary schools through the 1960s, at least for girls, according a 2010 study published in the Journal of the American Medical Association. These days, there are fewer and fewer such classes.
But all generations have reasons not to know as much about properly preparing food as educators might want.
“The silent generation grew up in a time where a lot less was known about proper food safety, preparation and handling,” Rumble said.
The new UF/IFAS study is published in the journal Food Control.
-30-
By: Brad Buck, 352-294-3303, bradbuck@ufl.eduSource: Joy Rumble, 352-273-1663, jnrumble@ufl.edu
Newswise —
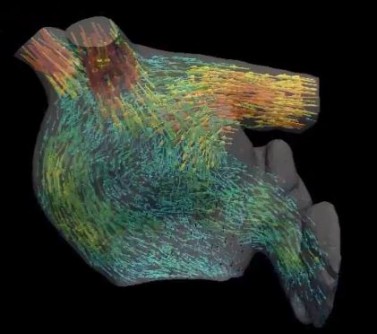
Using specialized CT scans of a healthy heart and one with heart disease, a team of Johns Hopkins cardiologists and biomedical engineers say they've created computer models of the "shape" of blood flow through the heart's upper left chamber that someday may help predict stroke risk.
Specifically, their computer visualizations found that blood in the diseased heart failed to flow in corkscrewlike "eddies" that most effectively moved blood out of the left atrium in the healthy heart and "showed us exactly how this motion would increase the risk of developing a blood clot," says Hiroshi Ashikaga, M.D., Ph.D., assistant professor of medicine and member of the Heart and Vascular Institute at the Johns Hopkins University School of Medicine.
The researchers say the same fluid motion analysis used in their two-heart proof-of-concept study may one day offer an accurate way to predict stroke risk in people with heart disease marked by enlargement and weakness of the cardiac muscle.
A description of the study and its results was published in the November print issue ofAnnals of Biomedical Engineering.
"By looking at blood flow through the atrium, we think we can accurately assess stroke risk better than such risk factors as heart size and pumping strength," says Ashikaga. "Our study fills in a missing diagnostic link between heart function and fluid motion in our understanding of how each can affect stroke risk."
Before this study, Ashikaga notes, researchers knew that enlargement of the heart, particularly the left upper chamber, was linked to increased stroke risk, particularly in people with atrial fibrillation, an irregular and often very rapid heart rate. Heart disease experts estimate that more than 1.6 million Americans each year are diagnosed with symptoms of atrial fibrillation that put them at risk for strokes caused by blood that pools in the heart and forms a clot, then travels to the brain.
The new study, Ashikaga says, sheds significant light on just how an enlarged and "floppy" atrium led to blood clot formation.
To collect the data needed to create the blood flow models, the Johns Hopkins team recruited two patients with a history of atrial fibrillation -- a 58-year-old woman with a healthy heart and a 68-year-old man with an enlarged heart. Each underwent a CT scan of their heart.
Using the images, the researchers then computed the movement of blood flow as it entered the left atrium from the pulmonary veins, then passed through a valve into the left lower chamber, or ventricle. Finally, they fashioned a video representation of the fluid motion of the blood.
In visualizing the healthy heart, the researchers saw that the blood flow formed into tight, corkscrewlike motions that circled around into doughnut formations, known as vortexes. The researchers say the vortexes helped move the blood efficiently through the atrium quicker and with less contact with the atrium's surface tissue. See blood flow modelled in ahealthy heart here.
The diseased heart they chose to examine was enlarged due to overuse, muscle fatigue and scarring, all of which can promote atrial fibrillation.
In the enlarged heart, the researchers noticed that at the top of the atrium, the blood never fully forms the corkscrews that loop around into vortexes. Instead, by the time the blood reaches the bottom of the atrium, it seems to be falling in "sheets" that coat the surface of the heart. See blood flow through a diseased heart here.
"As the blood comes in contact with the atrium's surface, it slows down due to shearing forces similar to friction, and this appears to prevent the blood from exiting the chamber as smoothly as it might," says Ashikaga. "The slower the blood moves and the more contact it has with the atrium, the more risk there is for a clot to form."
Ashikaga says his team is currently conducting a larger long-term study looking at the blood flow of many more people with normal and ailing hearts, and monitoring the incidence of stroke and other signs of blood clots over time.
He also hopes to develop the CT scan and computer analysis into a tool to predict stroke risk.
According to the Centers for Disease Control and Prevention, an estimated 3 million to 6 million people have atrial fibrillation and nearly 800,000 people have strokes each year in the United States. The most common symptom of stroke is numbness or weakness on one side of the body. Physicians use CT scans of the brain, blood tests, EKGs, MRI scans or other imaging test to determine if a person has suffered from a stroke. Strokes may be treated with clot-busting drugs, blood thinners and sometimes surgery to remove the clot.
Additional authors of the study include Tomohiro Otani, Abdullah Al-Issa, Amir Pourmorteza and Elliot McVeigh of Johns Hopkins Medicine, and Shigeo Wada of Osaka University.
The research was funded by grants from the Japan Society for the Promotion of Science, Magic That Matters Fund for Cardiovascular Research and the Zegar Family Foundation.
Newswise —
If you were told that there is a silent killer in the U.S. that takes the lives of up to half the people who get it each year—more than 250,000 people—would you know what it is? If you were told that it can strike anyone of any age and kills more people than prostate cancer, breast cancer and AIDS combined, would that narrow it down?
According to medical experts, if you have no idea what the condition is, you’re like most Americans. Most people have never heard of sepsis, have no idea what it is, and don’t know what they can do about it.
First, sepsis is when the body has an overwhelming response to an infection. The infection can be in the lungs, urinary tract, skin, abdomen (such as appendicitis) or any other part of the body. Some people think that people only get sepsis from being in the hospital, but the majority of people who get sepsis develop the initial infection outside the hospital. Sepsis can develop from any infection, even a cut or a skin infection.
Once you have an infection, immune chemicals released into the blood to combat the infection cause tissue damage. In severe cases, one or more organs fail. In the worst cases, blood pressure drops, the heart weakens and the patient spirals toward septic shock. Once this happens, multiple organs—lungs, kidneys, liver—may quickly fail and the patient can die.One of the things you can do to prevent sepsis is to prevent infection.
1. Get vaccinated against the flu, pneumonia, and any other infections that could lead to sepsis. Talk to your doctor for more information.
2. Prevent infections that can lead to sepsis by cleaning scrapes and wounds right away, do not pick scabs and keep wounds clean and practicing good hygiene (e.g., hand washing for at least 15 seconds).
You can also prevent other people from acquiring infections by getting the flu vaccine, covering your mouth when you cough and staying home from work when you are sick or have a fever. You can learn more about how to prevent the spread of germs by visiting the CDC website.
People with weakened immune systems, such as HIV patients, the elderly, infants, or patients undergoing chemotherapy, should be extra cautious with preventing infections, as they are at higher risk of developing sepsis.
For more information about sepsis and protocols to stop the spread of sepsis, visit pinnaclehealth.org/sepsis.
Newswise — PHILADELPHIA—
Cellular senescence is a state in which normal healthy cells do not have the ability to divide. Senescence can occur when cancer-causing genes are activated in normal cells or when chemotherapy is used on cancer cells. Thus, senescence induces a mechanism that halts the growth of rapidly dividing cells. Once thought to only be beneficial to halt cancer progression, work from the The Wistar Institute has shown that during senescence there is an increase in secreted factors called cytokines and chemokines (small proteins important in immune responses) that may have detrimental, pro-tumorigenic side effects.
Researchers at The Wistar Institute have identified a protein that plays a critical role in the expression of cytokines and chemokines, and that decreasing this protein suppresses the expression of these secreted factors. This suggests that there may be ways of promoting the positive effects of senescence while suppressing its negative effects. The findings were published online by the Journal of Cell Biology.
Rugang Zhang, Ph.D., professor and co-program leader of the Gene Expression and Regulation program at Wistar, and colleagues focused on chromatin, a cellular structure responsible for holding the DNA in our cells together. During senescence, some of the chromatin is reorganized into senescence-associated heterochromatin foci (SAHF). When this happens, genes that are responsible for promoting proliferation are silenced. However, the expression of cytokine and chemokine genes – known collectively as the senescence-associated secretory phenotype (SASP) – is increased.
“When senescence happens, you have two closely linked phenomena occurring, yet one of these helps to halt tumor progression while the other causes an increase in potentially harmful inflammatory cytokines and chemokines,” said Zhang, who is lead author of the study. “We pinpointed the architecture of chromatin and the proteins that influence chromatin organization as the proper place to start to try and solve this paradox.”
The scientists looked at a set of proteins known as high mobility group proteins, which are responsible for altering chromatin architecture in order to regulate gene transcription. One such protein called high mobility group box 2 (HMGB2) binds to DNA to increase chromatin’s accessibility to transcription factors. They showed that HMGB2 promotes SASP gene expression by preventing the spreading of heterochromatin and therefore preventing SAHF from silencing SASP genes. When the researchers silenced HMGB2, SASP genes were successfully silenced by SAHF, suggesting that the detrimental effects of senescence might be negated by inhibiting HMGB2.
“Understanding senescence is critical for understanding how tumor growth can be successfully suppressed,” said Katherine Aird, Ph.D., a staff scientist in the Zhang lab and first author of the study. “With the information from this study, we may be able to increase the effectiveness of chemotherapeutic agents that are able to induce senescence by silencing HMGB2 and decreasing the expression of unwanted secreted factors.”
The authors would like to thank The Wistar Institute’s Genomics, Bioinformatics, and Molecular Screening Facilities. This work was supported by the National Institutes of Health/National Cancer Institute grants R01CA160331, R01CA163377, R01CA202919, K99CA194309, and K99CA194318, an Ovarian Cancer Research Fund Alliance Program Project Development Grant, The Jayne Koskinas & Ted Giovanis Breast Cancer Research Consortium at Wistar, the subsidy of the Russian Government to support the Program of competitive growth of Kazan Federal University, and the W.W. Smith Charitable Trust grant C1501. Support of Core Facilities used in this study was provided by Cancer Center Support Grant (CCSG) CA010815 to The Wistar Institute.
Co-authors of this study from The Wistar Institute include: Osamu Iwasaki, Andrew Kossenkov, Hideki Tanizawa, Nail Fatkhutdinov, Benjamin Bitler, Linh Le, Gretchen Alicea, and Ken-ichi Noma. Additional co-authors are: Ting Yang and F. Bradley Johnson, both from the University of Pennsylvania Perelman School of Medicine.
###
The Wistar Institute is an international leader in biomedical research with special expertise in cancer research and vaccine development. Founded in 1892 as the first independent nonprofit biomedical research institute in the country, Wistar has held the prestigious Cancer Center designation from the National Cancer Institute since 1972. The Institute works actively to ensure that research advances move from the laboratory to the clinic as quickly as possible. wistar.org.
Newswise —
Fine particulate matter air pollution may be associated with blood vessel damage and inflammation among young, healthy adults, according to new research inCirculation Research, an American Heart Association journal.
“These results substantially expand our understanding about how air pollution contributes to cardiovascular disease by showing that exposure is associated with a cascade of adverse effects,” said C. Arden Pope, Ph.D., study lead author and Mary Lou Fulton Professor of Economics at Brigham Young University in Provo, Utah.
“These findings suggest that living in a polluted environment could promote the development of high blood pressure, heart disease and stroke more pervasively and at an earlier stage than previously thought,” said Aruni Bhatnagar, Ph.D., study co-author and the Smith and Lucille Gibson Chair in Medicine at the University of Louisville. “Although we have known for some time that air pollution can trigger heart attacks or strokes in susceptible, high-risk individuals, the finding that it could also affect even seemingly healthy individuals suggests that increased levels of air pollution are of concern to all of us, not just the sick or the elderly.”
Air pollution is known to contribute to cardiovascular disease and related deaths. In 2004, the American Heart Association released a scientific statement, updated in 2010, warning of the risk and recommending that people talk to their doctor about avoiding exposure to air pollution specific to their area. What remained unclear, however, was how air pollution actually affects the blood vessels to increase the risk of disease.
For this study, investigators analyzed the component of air pollution known as fine particulate matter (PM2.5) — the tiny pieces of solid or liquid pollution emitted from motor vehicles, factories, power plants, fires and smoking. They found that periodic exposure to fine particulate matter was associated with several abnormal changes in the blood that are markers for cardiovascular disease. As air pollution rose, they found:• small, micro-particles indicating cell injury and death significantly increased in number;• levels of proteins that inhibit blood vessel growth increased; and• proteins that signify blood-vessel inflammation also showed significant increases.
Study participants included 72 healthy, nonsmoking, adults in Provo, Utah. Their average age was 23, most were white and more than half were male. During the winters of 2013, 2014 and 2015, participants provided blood samples, which researchers then tested for markers of cardiovascular disease. Due to the unique weather and geographical features of Provo, they were able to evaluate these informative blood markers with various levels of air pollution.
However, researchers noted that the third study year, 2015, was relatively unpolluted, which could have affected the results.
Other co-authors are James P. McCracken, Ph.D.; Wesley Abplanalp, Ph.D.; Daniel J. Conklin, Ph.D.; and Timothy O’Toole, Ph.D., all of UofL. The National Institutes of Health funded the study.
Additional Resources:• Follow AHA/ASA news on Twitter @HeartNews• For updates and new science from the Circulation: Research journal follow @CircRES
###
Newswise —
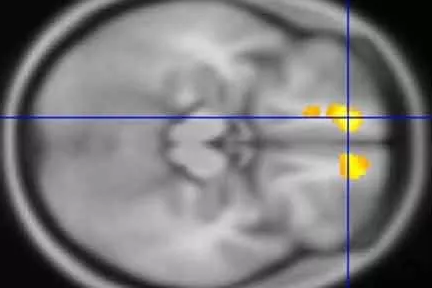
Using MRIs, researchers at Washington University School of Medicine in St. Louis have identified areas in the brains of children with Tourette’s syndrome that appear markedly different from the same areas in the brains of children who don’t have the neuropsychiatric disorder.
The findings are available online in the journal Molecular Psychiatry.
Tourette’s syndrome is defined by tics — involuntary, repetitive movements and vocalizations. Scientists estimate that the condition affects roughly one to 10 kids out of every 1,000 children.
“In this study, we found changes primarily in brain regions connected to sensation and sensory processing,”said co-principal investigator Kevin J. Black, MD, a professor of psychiatry.
Differences in those brain regions make sense, Black said, because many people with Tourette’s explain that their tics occur mainly as a response to unusual sensations. The feeling that a part of the body doesn’t seem right, for example, prompts an involuntary sigh, vocalization, cough or twitch.
“Just as you or I might cough or sneeze due to a cold, a person with Tourette’s frequently will have a feeling that something is wrong, and the tic makes it feel better,” Black said. “A young man who frequently clears his throat may report that doing so is a reaction to a tickle or some other unusual sensation in his throat. Or a young woman will move her shoulder when it feels strange, and the movement, which is a tic, will make the shoulder feel better.”
In the largest study of its kind, the researchers conducted MRI scans at four U.S. sites to study the brains of 103 children with Tourette’s and compared them with scans of another 103 kids of the same age and sex but without the disorder. The scans of the children with Tourette’s revealed significantly more gray matter in the thalamus, the hypothalamus and the midbrain than in those without the disorder.
The gray matter is where the brain processes information. It’s made up mainly of cells such as neurons, glial cells and dendrites, as well as axons that extend from neurons to carry signals.
In kids with Tourette’s, the researchers also found less white matter around the orbital prefrontal cortex, just above the eyes, and in the medial prefrontal cortex, also near the front, than in kids without the condition.
White matter acts like the brain’s wiring. It consists of axons that — unlike the axons in gray matter — are coated with myelin and transmit signals to the gray matter. Less white matter could mean less efficient transmission of sensations, whereas extra gray matter could mean nerve cells are sending extra signals.
Black said it’s not possible to know yet whether the extra gray matter is transmitting information that somehow contributes to tics or whether reduced amounts of white matter elsewhere in the brains of kids with Tourette’s may somehow influence the movements and vocalizations that characterize the disorder. But he said that discovering these changes in the brain could give scientists new targets to better understand and treat Tourette’s.
“This doesn’t tell us what happened to make the brain look this way,” Black explained. “Are there missing cells in certain places, or are the cells just smaller? And are these regions changing as the brain tries to resist tics? Or are the differences we observed contributing to problems with tics? We simply don’t know the answers yet.”
Black said the researchers will aim to replicate these findings in additional patients and determine if and how the brain regions they identified may contribute to Tourette’s syndrome, with a goal of developing more effective therapies.
Green, DJ, Williams AC, Koller JM, Schlaggar BL, Black KJ, and the Tourette Association of America Neuroimaging Consortium. Brain structure in pediatric Tourette syndrome. Molecular Psychiatry.
This work was supported by the National Institute of Mental Health, the National Cancer Institute, the Eunice Kennedy Shriver National Institute of Child Health & Human Development, and the National Institute of Neurological Disorders and Stroke of the National Institutes of Health (NIH), grant numbers K24 MH087913, P30 CA091842, P50 MH077248, UL1 TR000448, K01 MH104592, R21 NS091635, U54 HA087011. Additional funds came from the Tourette Association of America and its donors.
Washington University School of Medicine‘s 2,100 employed and volunteer faculty physicians also are the medical staff of Barnes-Jewish and St. Louis Children’s hospitals. The School of Medicine is one of the leading medical research, teaching and patient-care institutions in the nation, currently ranked sixth in the nation by U.S. News & World Report. Through its affiliations with Barnes-Jewish and St. Louis Children’s hospitals, the School of Medicine is linked to BJC HealthCare.
FOR IMMEDIATE RELEASE
Newswise — Studying brain tissue from deceased donors, Johns Hopkins scientists have found common groups of genes disrupted among people with schizophrenia, bipolar disorder and major depression. The commonly affected genes sets, identified with RNA sequencing methods, engage in making proteins, controlling brain cell communications and mounting an immune system response, the researchers say.
"There are subtle differences in individual genes, and these differences are enriched in sets of genes involved in specific cell processes in the brain tissue of people with a variety of severe mental disorders," says Sarven Sabunciyan, Ph.D., assistant professor of pediatrics and researcher in the Stanley Division of Developmental Neurovirology at the Johns Hopkins University School of Medicine. "It was striking to us that we could identify the broad functional overlaps, knowing there is a lot of variability among individuals with mental disorders."
"It is important to show what these seemingly disparate diseases have in common, not only to learn more about them, but because common deficits could suggest similar treatment strategies," says Miranda Darby, Ph.D., a research fellow in Sabunciyan's laboratory and the first author on the study.
A report on the work was published online Sept. 13 in Translational Psychiatry.
For the study, the researchers took 100 tissue samples from donor brains gathered by the Stanley Medical Research Institute's (SMRI) Array Collection. All samples were from the hippocampus -- the seahorse-shaped part of the brain important for memory and spatial navigation. Thirty-five brains were from people with schizophrenia, 33 were from people with bipolar disorder and 32 were controls without a mental disorder.
The research team also used 57 samples from a region of the brain's outer cortex near the eye, the orbitofrontal cortex, all gathered by SMRI's Neuropathy Consortium to verify that the findings in one part of the brain replicated in another part. Thirteen of those brain samples were from people with schizophrenia, 14 with bipolar disorder, 15 with major depression and 15 from controls.
Of the total brain samples from both the hippocampus and the orbitofrontal cortex, 57 were from women and 100 were from men. All but seven samples were from Caucasians, and the donors' ages ranged from 19 to 68 at the time of death.
The researchers extracted and sequenced the mRNA -- genetic material that function as the blueprints created from DNA and used as guides by cells to build proteins -- from the tissue. The investigators report that each sample from the hippocampus produced on average 154 million sequenced bits of RNA and 140 million sequences for each brain sample from the orbitofrontal cortex. They then aligned the sequences from each sample with a fully sequenced human genome (version 19) and counted the number of times a sequence matched up to each individual gene. In all, 21,861 genes were represented in the hippocampus tissues, and 20,711 were represented in the orbitofrontal cortex region tissues.
The researchers identified genes that make either more or less mRNA in individuals with mental disorders than in individuals without a mental disorder. They then compared the list of genes affected in each disorder to lists of genes grouped by their function in the cell, and identified which groups contained a disproportionate number of genes with either increased or decreased mRNA in individuals with schizophrenia, bipolar disorder and major depression.
The team reports that out of a total of 1,070 gene sets, 13 of these groups changed in common ways among all three mental disorders.
Of these, nine groups containing a total of 338 genes included ribosomal genes, genes responsible for making proteins. A subset of 80 of those protein-making genes was in each of the nine sets, and most of these, the team says, were turned on, or activated, at higher levels compared to controls. For example, in samples from brains of people with bipolar disorder, 78 of these genes were turned on higher in the hippocampus, and 79 were turned on higher in the orbitofrontal cortex than controls. In both regions of the brain, samples from people with major depression had 78 of the genes turned up higher than controls. In people with schizophrenia, 56 genes were turned up in the hippocampus, and 52 were turned up in the orbitofrontal cortex higher than the controls.
"Although there isn't a clear reason why the brains of people with these mental disorders would have more of the protein production machinery, we think our findings suggest that it's a fruitful line of investigation to pursue," says Sabunciyan. The remaining four gene groups in common among the brain tissue from all three sets of those with mental illness were "turned down" compared to the controls, the team reported. Two of the gene sets have clear roles in how the brain's neurons send and receive messages to neighboring cells, with one set making up general neuron genes and the other specific to GABA, a neurotransmitter used as the actual messenger between neurons.
Another gene group is believed to help operate the ways in which the immune system mounts a response to foreign -- and potentially threatening -- antigens, biological molecules presented by a virus, bacterium or parasite. The researchers say that disruption of the immune system is a hallmark feature long observed in some people with mental illness, particularly in schizophrenia and, to a lesser extent, in bipolar disorder.
The fourth gene set included genetic components involved in endocytosis -- the process used to engulf biological molecules outside the cell and bring them inside. When neurons suck up neurotransmitters for recycling after they have been sent or received as a message, they use endocytosis. Endocytosis also plays a role in the immune system's process of engulfing and disposing of germs.
Sabunciyan says the research team plans to study these changes in induced pluripotent stem cells derived from patients, which also show an increase in ribosomal gene expression.
According to the National Institute of Mental Health, approximately 1 percent of adults develop schizophrenia, about 2.5 percent have bipolar disorder and almost 7 percent of people develop major depression over their lifetimes.
Robert Yolken, M.D., the Theodore and Vada Stanley Distinguished Professor of Neurovirology in Pediatrics at Johns Hopkins, is an additional author on the study.
This study was funded by the nonprofit Stanley Medical Research Institute.
Newswise —
NEW YORK, NY —
Pregnancy was not found to raise the risk of stroke in older women, according to a study from Columbia University Medical Center and NewYork-Presbyterian. In younger women, however, the risk of stroke was significantly higher for those who were pregnant.
The researchers published their findings today in JAMA Neurology.
Pregnancy-associated stroke occurs in an estimated 34 out of 100,000 women. Previous studies suggested that the risk of pregnancy-associated stroke is higher in older women than in younger women.
“The incidence of pregnancy-associated strokes is rising, and that could be explained by the fact that more women are delaying childbearing until they are older, when the overall risk of stroke is higher,” said Joshua Z. Willey, MD, assistant professor of neurology at CUMC, assistant attending neurologist on the stroke service at NewYork-Presbyterian/Columbia, and a senior author on the paper. “However, very few studies have compared the incidence of stroke in pregnant and non-pregnant women who are the same age.”
In this study, the researchers examined data collected on every woman hospitalized for stroke in New York State between 2003 and 2012. Of these 19,146 women, age 12 to 55 years, 797 (4.2 percent) were pregnant or had just given birth.
The researchers found that the overall incidence of stroke during or soon after pregnancy increased with age (46.9 per 100,000 in women age 45 to 55 vs 14 per 100,000 in women age 12 to 24).
However, pregnant and postpartum women in the youngest group (age 12 to 24) had more than double the risk of stroke than non-pregnant women in the same age group (14 per 100,000 in pregnant women vs 6.4 in non-pregnant women).
For women age 25 to 34, pregnancy increased the risk 1.6 times. Stroke risk was similar in pregnant and non-pregnant women in the older age groups.
“We have been warning older women that pregnancy may increase their risk of stroke, but this study shows that their stroke risk appears similar to women of the same age who are not pregnant,” said Eliza C. Miller, MD, a vascular neurology fellow in the Department of Neurology at CUMC and NewYork-Presbyterian and lead author of the study. “But in women under 35, pregnancy significantly increased the risk of stroke. In fact, 1 in 5 strokes in women from that age group were related to pregnancy. We need more research to better understand the causes of pregnancy-associated stroke, so that we can identify young women at the highest risk and prevent these devastating events.”
The study is titled “Risk of Pregnancy-Associated Stroke Across Age Groups in New York State.” Authors included Eliza C. Miller (Columbia University Medical Center and NewYork-Presbyterian, New York, NY), Hajere J. Gatollari (CUMC), Gloria Too (CUMC and NewYork-Presbyterian), Amelia K. Boehme (CUMC), Lisa Leffert (Massachusetts General Hospital, Boston, MA), Mitchell S. V. Elkind (CUMC and NewYork-Presbyterian), and Joshua Z. Willey (CUMC and NewYork-Presbyterian).
The study was supported by grants from the National Institute of Neurological Disorders and Stroke (T32 NS007153-31 and K23 073104).
Additional financial disclosures are included in the study.
###
Newswise —
To infect its victims, influenza A heads for the lungs, where it latches onto sialic acid on the surface of cells. So researchers created the perfect decoy: A carefully constructed spherical nanoparticle coated in sialic acid lures the influenza A virus to its doom. When misted into the lungs, the nanoparticle traps influenza A, holding it until the virus self-destructs.
In a study on immune-compromised mice, the treatment reduced influenza A mortality from 100 percent to 25 percent over 14 days. The novel approach, which is radically different from existing influenza A vaccines, and treatments based on neuraminidase inhibitors, could be extended to a host of viruses that use a similar approach to infecting humans, such as Zika, HIV, and malaria. Results were published today in the advanced online edition of the journal Nature Nanotechnology.
“Instead of blocking the virus, we mimicked its target – it’s a completely novel approach,” said Robert Linhardt, a glycoprotein expert and Rensselaer Polytechnic Institute professor who led the research. “It is effective with influenza and we have reason to believe it will function with many other viruses. This could be a therapeutic in cases where vaccine is not an option, such as exposure to an unanticipated strain, or with immune-compromised patients.”
The project is a collaboration between researchers within the Center for Biotechnology and Interdisciplinary Studies (CBIS)at Rensselaer and several institutions in South Korea including Kyungpook National University. Lead author Seok-Joon Kwon, a CBIS research scientist, coordinated the project across borders, enabling the South Korean institutions to test a drug designed and characterized at Rensselaer. Authors included Kwon, Linhardt, Ravi S. Kane, Jonathan S. Dordick, Marc Douaisi, and Fuming Zhang at Rensselaer; and Korean researchers Kyung Bok Lee, Dong Hee Na, Jong Hwan Kwak, Eun Ji Park, Jong-Hwan Park, Hana Youn, and Chang-Seon Song.
To access the interior of a cell and replicate itself, influenza A must first bind to the cell surface, and then cut itself free. It binds with the protein hemagglutinin, and severs that tie with the enzyme neuraminidase. Influenza A produces numerous variations each of hemagglutinin and neuraminidase, all of which are antigens within the pathogen that provoke an immune system response. Strains of influenza A are characterized according to the variation of hemagglutinin and neuraminidase they carry, thus the origin of the familiar H1N1 or H3N2 designations.
Medications to counter the virus do exist, but all are vulnerable to the continual antigenic evolution of the virus. A yearly vaccine is effective only if it matches the strain of virus that infects the body. And the virus has shown an ability to develop resistance to a class of therapeutics based on neuraminidase inhibitors, which bind to and block neuraminidase.
The new solution targets an aspect of infection that does not change: all hemagglutinin varieties of influenza A must bind to human sialic acid. To trap the virus, the team designed a dendrimer, a spherical nanoparticle with treelike branches emanating from its core. On the outermost branches, they attached molecules, or “ligands,” of sialic acid.
The research found that the size of the dendrimer and the spacing between the ligands is integral to the function of the nanoparticle. Hemagglutinin occurs in clusters of three, or “trimers,” on the surface of the virus, and researchers found that a spacing of 3 nanometers between ligands resulted in the strongest binding to the trimers. Once bound to the densely packed dendrimer, viral neuraminidase is unable to sever the link. The coat of the virus contains millions of trimers, but the research revealed that only a few links provokes the virus to discharge its genetic cargo and ultimately self-destruct.
A different approach, using a less structured nanoparticle, had been previously tested in unrelated research, but the nanoparticle selected proved both toxic, and could be inactivated by neuraminidase. The new approach is far more promising.
“The major accomplishment was in designing an architecture that is optimized to bind so tightly to the hemagglutinin, the neuraminidase can’t squeeze in and free the virus,” said Linhardt. “It’s trapped.”
“Nanostructured glycan architecture is important in the inhibition of influenza A virus infection” appears in the Advance Online Publication (AOP) published today on Nature Nanotechnology's website. The Digital Object Identifer for this paper is 10.1038/nnano.2016.181.
At Rensselaer, this research fulfills the vision of The New Polytechnic, an emerging paradigm for higher education, which recognizes that global challenges and opportunities are so complex, they cannot be addressed by even the most talented person working alone. Rensselaer serves as a crossroads for collaboration — working with partners across disciplines, sectors, and geographic regions, to address global challenges — and addresses some of the world’s most pressing technological challenges, from energy security and sustainable development to biotechnology and human health. The New Polytechnic is transformative in the global impact of research, in its innovative pedagogy, and in the lives of students at Rensselaer.
Newswise —
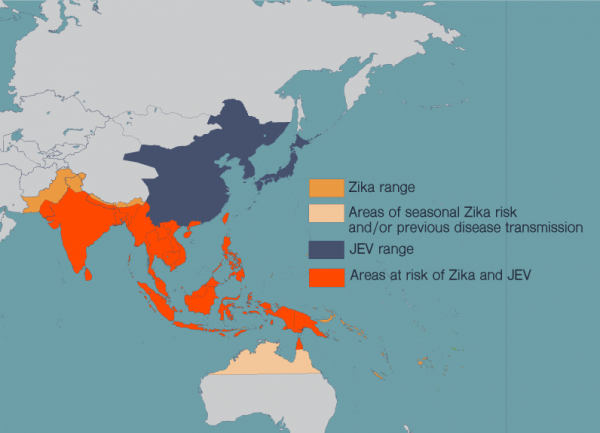
La Jolla, CA- Concerns over the Zika virus have focused on pregnant women due to mounting evidence that it causes brain abnormalities in developing fetuses. However, new research in mice from scientists at The Rockefeller University and La Jolla Institute for Allergy and Immunology suggests that certain adult brain cells may be vulnerable to infection as well. Among these are populations of cells that serve to replace lost or damaged neurons throughout adulthood, and are also thought to be critical to learning and memory.
“This is the first study looking at the effect of Zika infection on the adult brain,” says Joseph Gleeson, adjunct professor at Rockefeller and head of the Laboratory of Pediatric Brain Disease and Howard Hughes Medical Institute investigator. “Based on our findings, getting infected with Zika as an adult may not be as innocuous as people think.”
Although more research is needed to determine if this damage has long-term biological implications or the potential to affect behavior, the findings suggest the possibility that the Zika virus, which has become widespread in Central and South America over the past eight months, may be more harmful than previously believed.
“Zika can clearly enter the brain of adults and can wreak havoc,” says Sujan Shresta, a professor at the La Jolla Institute of Allergy and Immunology. “But it’s a complex disease—it’s catastrophic for early brain development, yet the majority of adults who are infected with Zika rarely show detectable symptoms. Its effect on the adult brain may be more subtle, and now we know what to look for.”
Neuronal progenitorsEarly in gestation, before our brains have developed into a complex organ with specialized zones, they are comprised entirely of neural progenitor cells. With the capability to replenish the brain’s neurons throughout its lifetime, these are the stem cells of the brain.In healthy individuals, neural progenitor cells eventually become fully formed neurons, and it is thought that at some point along this progression they become resistant to Zika, explaining why adults appear less susceptible to the disease.
But current evidence suggests that Zika targets neural progenitor cells, leading to loss of these cells and to reduced brain volume. This closely mirrors what is seen in microcephaly, a developmental condition linked to Zika infection in developing fetuses that results in a smaller-than-normal head and a wide variety of developmental disabilities.
The mature brain retains niches of these neural progenitor cells that appear to be especially impacted by Zika. These niches—in mice they exist primarily in two regions, the subventricular zone of the anterior forebrain and the subgranular zone of the hippocampus—are vital for learning and memory.
Gleeson and his colleagues suspected that if Zika can infect fetal neural progenitor cells, it wouldn’t be a far stretch for them to also be able to infect these cells in adults. In a mouse model engineered by Shresta and her team to mimic Zika infection in humans, fluorescent biomarkers illuminated to reveal that adult neural progenitor cells could indeed be hijacked by the virus.
“Our results are pretty dramatic – in the parts of the brain that lit up, it was like a Christmas tree,” says Gleeson. “It was very clear that the virus wasn’t affecting the whole brain evenly, like people are seeing in the fetus. In the adult, it’s only these two populations that are very specific to the stem cells that are affected by virus. These cells are special, and somehow very susceptible to the infection.”
Beyond fetal brain infectionThe researchers found that infection correlated with evidence of cell death and reduced generation of new neurons in these regions. Integration of new neurons into learning and memory circuits is crucial for neuroplasticity, which allows the brain to change over time. Deficits in this process are associated with cognitive decline and neuropathological conditions, such as depression and Alzheimer’s disease.
Gleeson and colleagues recognize that healthy humans may be able to mount an effective immune response and prevent the virus from attacking. However, they suggest that some people, such as those weakened immune systems, may be vulnerable to the virus is a way that has not yet been recognized.
“In more subtle cases, the virus could theoretically impact long-term memory or risk of depression,” says Gleeson, “but tools do not exist to test the long-term effects of Zika on adult stem cell populations.”
In addition to microcephaly, Zika has been linked to Guillain-Barré syndrome, a rare condition in which the immune system attacks parts of the nervous system, leading to muscle weakness or even paralysis. “The connection has been hard to trace since Guillain-Barré usually develops after the infection has cleared,” says Shresta. “We propose that infection of adult neural progenitor cells could be the mechanism behind this.”
There are still many unanswered questions, including exactly how translatable findings in this mouse model are to humans. Gleeson’s findings in particular raise questions such as: Does the damage inflicted on progenitor cells by the virus have lasting biological consequences, and can this in turn affect learning and memory? Or, do these cells have the capability to recover? Nonetheless, these findings raise the possibility that Zika is not simply a transient infection in adult humans, and that exposure in the adult brain could have long-term effects.
“The virus seems to be traveling quite a bit as people move around the world,” says Gleeson. “Given this study, I think the public health enterprise should consider monitoring for Zika infections in all groups, not just pregnant women.”
Joseph Gleeson also holds appointments at the University of California San Diego and Rady Children’s Hospital. This research was supported by the NIH R01NS041537, R01NS048453, R01NS052455, P01HD070494, P30NS047101, the Simons Foundation Autism Research Initiative (SFARI), the HowardHughes Medical Institute, California Institute of Regenerative Medicine (J.G.G.) and NIH R01 AI116813 (S.S.) and Druckenmiller Fellowship from New York Stem Cell Foundation (H.L).The DOI and Cell.com link will be: 10.1016/j.stem.2016.07.019 and http://www.cell.com/cell-stem-cell/fulltext/S1934-5909(16)30214-4
About The Rockefeller UniversityThe Rockefeller University is the world’s leading biomedical research university and is dedicated to conducting innovative, high-quality research to improve the understanding of life for the benefit of humanity. Our 79 laboratories conduct research in neuroscience, immunology, biochemistry, genomics, and many other areas, and a community of 1,800 faculty, students, postdocs, technicians, clinicians, and administrative personnel work on our 14-acre Manhattan campus. Our unique approach to science has led to some of the world’s most revolutionary and transformative contributions to biology and medicine. During Rockefeller’s 115-year history, 24 of our scientists have won Nobel Prizes, 21 have won Albert Lasker Medical Research Awards, and 20 have garnered the National Medal of Science, the highest science award given by the United States.
About La Jolla InstituteLa Jolla Institute for Allergy and Immunology is dedicated to understanding the intricacies and power of the immune system so that we may apply that knowledge to promote human health and prevent a wide range of diseases. Since its founding in 1988 as an independent, nonprofit research organization, the Institute has made numerous advances leading towards its goal: life without disease®.